
Unlocking the Molecular Mysteries of Addiction and Depression
Addiction and Depression are mental health disorders with biological origins in the brain. They are also public health challenges with serious consequences. Globally, depression is the leading cause of disability, affecting up to 15 percent of the population, while addiction’s devastating effects have made headlines across the globe and especially in the United States in relation to the opioid crisis. Unfortunately, today’s treatments for addiction and depression are inadequate for many patients. Continued basic research is needed in order to better understand these disorders and formulate more effective therapeutics.
“We have learned an enormous amount about how the brain functions under normal conditions at the molecular, cellular, and circuit levels and what goes wrong in individual syndromes, addiction and depression among them.” — Eric Nestler, Nash Family Professor of Neuroscience and Director of the Friedman Brain Institute at the Icahn School of Medicine at Mount Sinai
SfN Past President Eric Nestler has spent the last 30 years studying how the brain changes at the molecular level in response to addiction and depression. Nestler, Nash Family Professor of Neuroscience and director of the Friedman Brain Institute at the Icahn School of Medicine at Mount Sinai, uses animal models of these disorders to determine how drugs of abuse or stress change the brain, leading to addiction or depression-like behaviors.
Nestler studies addiction and depression together because they both involve key emotional centers in the brain called reward pathways. These brain regions, including the nucleus accumbens and ventral tegmental area, control mood, motivation, and sense of well-being. Nestler’s research shows that these reward pathways change over time in response to drug abuse and stress, resulting in the dramatic alterations in motivation and reward processing that characterize both addiction and depression.
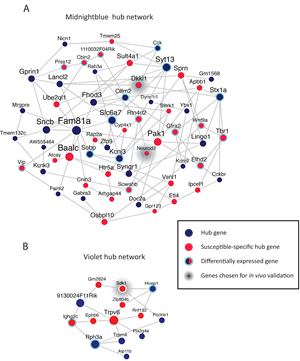
Like most psychiatric disorders, addiction and depression are defined behaviorally: There is no objective laboratory measure, such as a blood test or brain scan, with which to diagnose patients or determine a course of treatment for these disorders. The animal models in the Nestler Laboratory, however, accurately replicate much of the human addiction syndrome. If given the opportunity, animals will self-administer drugs and a subset will become addicted, choosing to take drugs at the cost of eating and socializing. More challenging is determining whether a mouse is depressed. Nestler uses stress-induced models of depression, in which mice or rats are exposed to periods of social or other types of stress; some of the stressed animals will develop behaviors that resemble human depression, such as not eating, sleeping, and interacting with other animals normally.
Using these powerful models, Nestler has been able to home in on themolecular changes that drugs and stress induce in the brain’s reward pathways and how these changes contribute to the behavioral symptoms of addiction and depression.
An important component of Nestler’s work has been examining not only the mice that succumb to stress but also those that are resistant to it. This recapitulates the human condition, as most individuals function normally despite exposure to severe stress. He has identified molecular, cellular, and circuit-level changes that underlie resilience to stress. These findings could lead to novel approaches to developing antidepressant medications, such as through promoting resilience rather than simply opposing the harmful effects of stress.
“The advances being made at both the molecular and the circuit level are making it possible to bridge the gap between these two dimensions of brain function. We will establish a more complete understanding of the brain and its diseases so that we can do a far better job of mining that information for more effective treatments for our patients.” — Eric Nestler
Another contribution of Nestler’s work has been his pioneering use of a technique known as viral-mediated gene transfer to turn genes on and off in specific cells of an adult animal’s brain. This method involves the use of recombinant viruses to express a protein in certain cell types in a localized brain area. “Using this technique, we were able to provide causal evidence that individual genes and proteins acting in a specific cell type are crucial in addiction-and depression-related phenomena,” Nestler said.

Additionally, Nestler has helped to demonstrate the important role of epigenetics in mediating addiction and depression. Epigenetic changes occur when an environmental stimulus, such as drug abuse or stress, produces long-lasting changes in the way an organism’s genetic code is expressed. Behavioral experience could create such permanent changes in the brain and be a contributing factor to the lifelong consequences of many psychiatric disorders.
“We have provided evidence that some of the same types of epigenetic modifications that control cell differentiation during development or cell transformation in cancer are involved in analogous ways in mediating the long-lasting effects of drugs of abuse and stress on the brain and behavior,” Nestler said.
Nestler believes that an improved understanding of the molecular changes that occur in addiction and depression will ultimately lead to innovative therapies that address the changes in the brain that drive addictive and depressive behaviors. “We have learned an enormous amount about how the brain functions under normal conditions at the molecular, cellular, and circuit levels and what goes wrong in individual syndromes, addiction and depression among them,” he said.
The challenge will be to translate that knowledge into the clinic — in addition to the molecular changes that take place in individual brain cells, one must also consider the billions of connections among them.
“Attacking the molecular abnormalities in one or a few types of brain cells will not be enough. To understand these syndromes, one also has to tackle the associated circuitry,” Nestler said. Still, he remains optimistic.
“The advances being made at both the molecular and the circuit level are making it possible to bridge the gap between these two dimensions of brain function. We will establish a more complete understanding of the brain and its diseases so that we can do a far better job of mining that information for more effective treatments for our patients.”























