In Review: Investing in the Brain’s Cognitive Reserve
New research shows how establishing healthy habits early in life may help delay age-related cognitive decline.
As life expectancy increases in many parts of the world, maintaining brain health throughout the lifespan becomes increasingly important. Although the aging brain is affected by forces outside one’s control — such as genetics and environmental hazards — a growing body of research published in JNeurosci and eNeuro suggests that experiences in youth, including diet, exercise, and sleep pattern, can pave the way for brain health in adulthood and beyond.
Nutrition and Exercise’s Impacts on Memory and Brain Health
Diet and exercise have been consistently shown to impact brain health in aging (1, 2). A study published in eNeuro by Olga Shevtsova et al. (3) found that adolescent rats given the opportunity to run on a wheel in their cages showed greater activation in adult-generated neurons in response to testing later in life than rats housed without wheels. The animals also had better memory as they aged, as well as enhanced performance on a fear response task known to be related to hippocampal neurogenesis. The findings support the concept of neurogenic reserve, whereby practices early in life can help build up the brain’s capacity to stay flexible and resistant to cognitive decline later in life.
Teenagers do not always make the best nutritional choices, and this may have long-term consequences. A study by Antonia Manduca et al. (4), published in JNeurosci, found that omega-3 fatty acid deficiency early on can influence mood, memory, and even brain plasticity later in life. Feeding mice an omega-3-deficient diet beginning in adolescence was linked to deficits in cognitive function, mood and anxiety, and plasticity in two key brain regions, the medial prefrontal cortex and the nucleus accumbens, in adulthood.
In a recent eNeuro study using a mouse model of Alzheimer’s disease, V. Alexandra Moser and Christian J. Pike (5) showed that obesity can interact with genetics to affect Alzheimer’s disease risk. Mice fed a diet high in sugar and saturated fat for 12 weeks had more amyloid-beta deposition and glial cell activation if they were carriers of the higher risk APOE4 variant, but not the more common APOE3 version. This may suggest that APOE4 carriers are more susceptible to the effects of poor diet and excess body weight, making it even more important to maintain a healthy diet and weight with age.
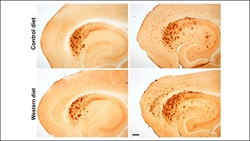
Carriers of the Alzheimer’s disease (AD) risk gene APOE4 may be more susceptible to the effects of diet-induced obesity on AD pathology. Credit: Moser and Pike, JNeurosci (2019).
Other work has suggested that it is not only what, but when we eat that affects brain health. In a mouse model of Huntington’s disease, Huei-Bin Wang et al. (6) demonstrated that young adult animals who were restricted to six-hour feeding periods for three months improved in motor function, heart rate variability, and genetic markers in the brain’s striatum, which is known to be affected in Huntington’s disease. The findings align with other work suggesting that time-restricted food intake can benefit metabolic measures, including blood pressure, weight, and insulin sensitivity, which are all important factors in both brain and heart health (7, 8).
High blood pressure, a risk factor for both heart and brain disease (9), appears to suppress the rate at which waste, including amyloid-beta, is cleared by the brain’s glymphatic system. In a 2019 study, Mortensen et al. (10) used MRI to track the flow of cerebral spinal fluid in hypertensive rats and found that glymphatic clearance was reduced in both early and late stage hypertension compared to rats without high blood pressure. Since glymphatic flow is dependent on arterial pulsatility, the authors suggest, it makes sense that compromised blood vessel function would alter clearance. These findings highlight the importance of maintaining healthy blood pressure throughout life.
The Permanent Effects of Sleep Quality
Much of the brain’s self-cleaning is done during sleep (11). A JNeurosci study by Yan Zhu et al. (12) focused on mice predisposed to developing the kind of tauopathy observed in Alzheimer’s disease. When these mice were subjected to disrupted sleep as adolescents, their brains showed increased tau protein earlier in life compared to their rested peers. Importantly, even when the mice were allowed extended periods of recovery time, increased tau was still present, which may suggest that damage early in life may not always be reversible. Interestingly, animals who had chronically fragmented sleep, rather than short sleep, showed similar results. The results suggest that adequate sleep starting early in life may be an important factor in cognitive and neurological health.
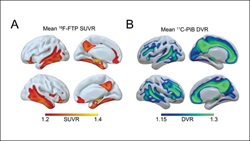
Sleep patterns can predict the accumulation of Alzheimer's pathology proteins later in life, which may allow for early preventive care. Credit: Winer et al., JNeurosci (2019).
Disrupted sleep appears to not only be a risk factor for Alzheimer’s disease in the future, but also one of its earliest symptoms. A study by Joseph R. Winer et al. (13) found that particular sleep pattern changes were linked to greater tau and amyloid-beta levels in humans, prompting the team to suggest that sleep patterns may also be used as a biomarker for Alzheimer’s risk, and that sleep interventions could be prescribed to delay its onset.
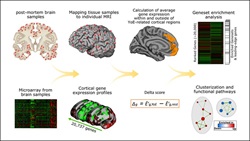
Genes and molecular pathways identified in a study of healthy older adults could provide insight into factors that help keep the brain sharp in old age. Credit: Bartrés-Faz et al., JNeurosci (2019).
The brain’s capacity to stay flexible with age has also been linked to formal education. David Bartrés-Faz et al. (14) recently identified anatomical and genetic evidence supporting this connection. The researchers found people who had 15 years or more of education had greater thickness in the frontal lobe’s anterior cingulate and orbital cortices, areas involved in memory and executive function. When researchers looked at the genetic profiles of these brain areas more closely, they saw increased expression of genes involved in synaptic transmission, or neuroplasticity, along with genes involved in immune function.
Taken together, these studies demonstrate that the aging brain is shaped by amendable lifestyle factors. Establishing healthy habits early in life may therefore help build the brain’s cognitive reserve and delay age-related decline.